Tempris – Product Temperature Measurement in Lyophilization
Product Temperature Measurement is the Most Important Parameter in Lyophilization
Product temperature measurement in Lyophilization is essential for the quality of the product and the optimization of the lyophilization cycle. As a PAT tool, our innovative wireless technology makes your production process cost-efficient and time-saving with high product quality. Tempris’ uninterrupted continuous process verification (CPV) guarantees reliable quality and optimum productivity.
Product Temperature Tp is the most critical parameter during the process of Lyophilization, but due to development in process optimization (e.g., automatic loading), it is not measured during the actual production on a regular base. Using the wireless Tempris system during this step allows you to
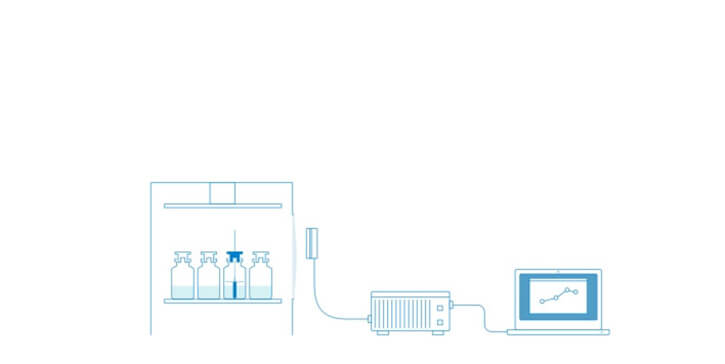
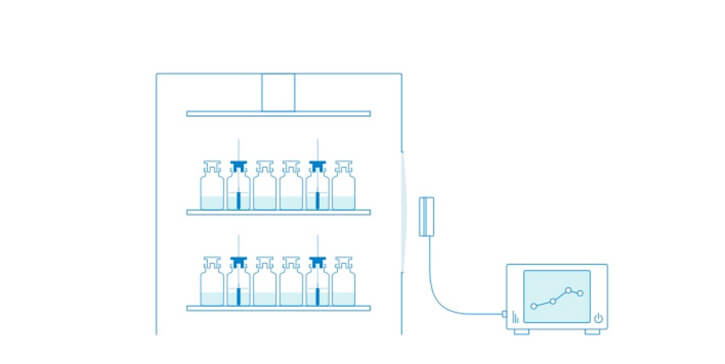
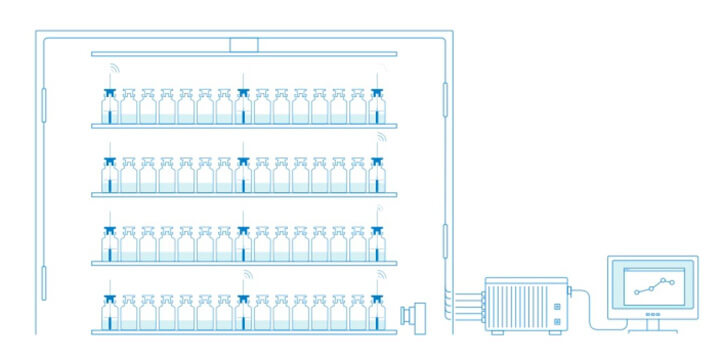
The same Tempris sensors used in laboratory scale can be used in pilot and production freeze-dryers under cGMP guidelines to obtain directly comparable data. Also, Tempris is ideally suited for integration into aseptic automated fill/finish production lines.
See how Tempris Sensor Technology Works
Fields of Application of the Tempris System
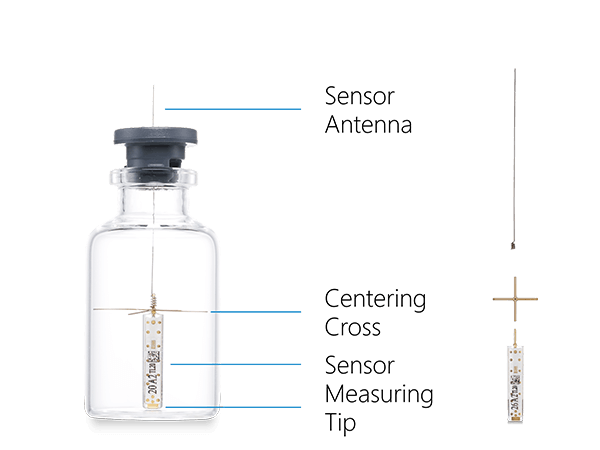
Sensor for Real-time Product Temperature Measurement
We specifically designed Tempris temperature sensors for the monitoring and optimization of freeze-drying processes. They have proven their reliability in many industrial and academic applications. Tempris sensors have a variety of advantages over other sensors measuring temperature in freeze drying
Tempris Technology for Product Temperature Measurement
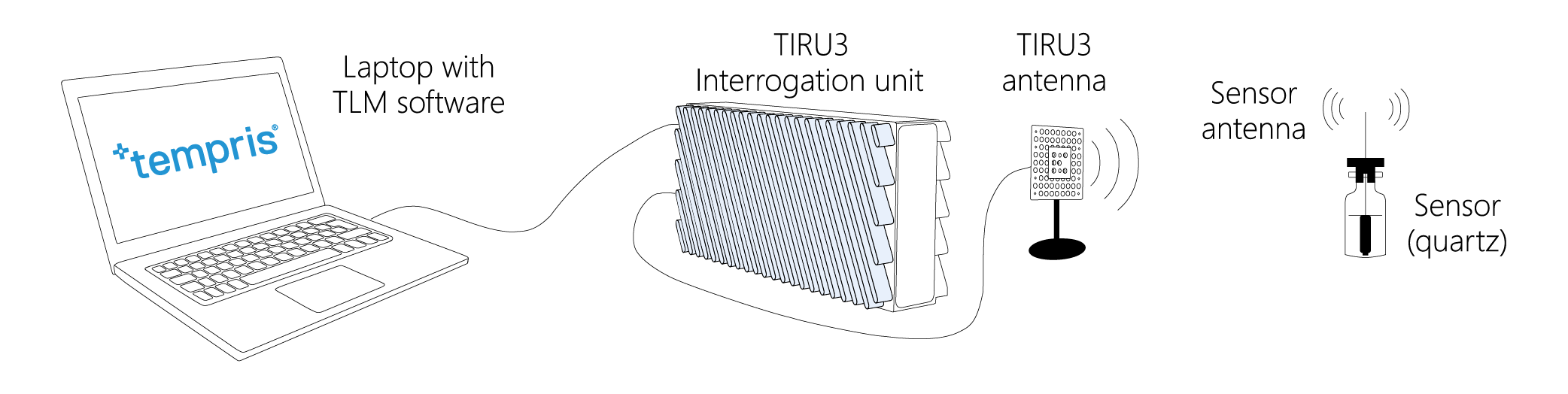
Tempris – Temperature remote interrogation system
The Tempris Lyophilization Monitoring Software TLM is used to control the interrogation unit (TIRU3), record data, set all parameters, visualize and export data. TLM gets the data of the Tempris Interrogation Unit TIRU3 which in turn gets temperature data from the antennas in the freeze-dryer. The antennas collect all the temperatures which are measured by the quartz based sensor (operating on the principle of temperature dependent resonance).
Pfizer’s Experience with Tempris
„Tempris wireless sensors allow placement of temperature sensors in any location within a freeze-dryer. They are also the sensor of choice if product temperatures of edge vials are monitored. Tempris sensors do not release heat while thermocouples or data loggers can transfer some heat into the product (very notable for edge vials), obscuring the actual product temperature. It is especially important if small fill volumes are used as readings of thermocouples and data loggers from the edge vials produce uncertain temperature profiles.”
Pfizer
Tempris Products – Product Temperature Measurement for all Lyo Scales
Lyo-cycle development in the laboratory establishes the base for the freeze drying cycle and subsequent optimization. When the product moves to a larger freeze-dryer, to a different type of freeze-dryer or simply to cGMP environment it was often necessary to change the Tp measurement technology. This causes problems or limitations with respect of the comparability of the obtained data. With Tempris, the same sensors which are used in lab scale development can also be used in pilot and production scale freeze-dryers at equivalent positions, thus providing directly comparable data.
Automated Sensor Loading by Robot
Fully automated aseptic sensor loading by robot according to GMP Annex 1 ensures the highest aseptic safety level. Each sensor is checked for the correct vial-size, identified and recorded for accurate process repetition and batch comparison. Pre-prepared vials with sensors are loaded automatically in the predefined critical positions.


Tempris Services
Tempris offers a wide range of additional services to easify your process and ensure regulatory issues. We accompany you from the decision to measure temperature, through installation and maintenance, to optimising your freeze-drying process with accurate temperature data. Our service and support includes: